The West African Ebola crisis which peaked in 2014 was a near-miss pandemic. Given the slowness of international financial assistance with disease control, it might have been much worse.
In August 2015, I was a panelist at a workshop on pandemic financing at the National Academy of Medicine (NAM) in Washington D.C. At that time, formal trials of an Ebola vaccine were about to begin.
Tragically, these came too late for the 11,000 victims of this virus; finance is a key factor in timely vaccine development. In 2016, there was a dearth of funding for human clinical trials for coronavirus vaccines. If COVID-19 had emerged two years earlier, the commercial board of BioNTech would not have considered the idea of building the mRNA vaccine.
In November 2021, the NAM released a report providing recommendations on leveraging COVID-19 vaccine technology for flu vaccine research and development. A priority focus for future research should overcome issues associated with the variable effectiveness of seasonal vaccines.
The NAM report recognized that a universal flu vaccine would be a game-changer in the mitigation of pandemic risk.
Even though a universal flu vaccine might not be available for a decade, it is possible to envisage faster progress. So important is a universal flu vaccine for medium-term pandemic risk management, that significant progress needs to be tracked, alongside any delays due to funding restrictions.
Just one year later, on November 25, 2022, a new milestone on the path to a universal flu vaccine has been established with the publication of a paper in the journal Science, describing a vaccine capable of protecting against all the known 20 flu subtypes.
This new approach to universal vaccine design is based on messenger RNA (mRNA) therapeutics. Safe, effective, and stable delivery systems are required to provide mRNA with protection from degradation.
The successful delivery of mRNA is achieved through lipid nanoparticles, as effectively demonstrated by their clinical use in the Pfizer-BioNTech and Moderna COVID-19 vaccines. Low temperatures are needed to stabilize the lipid nanoparticles until they are ready for injection at the time of vaccination.
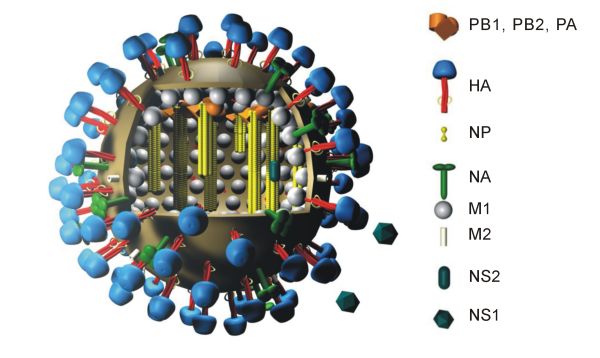
The new flu vaccine also uses mRNA–lipid nanoparticle technology. Using what the authors admit is a brute-force approach, representative hemagglutinin (HA) molecules of each of the known 20 flu subtypes were chosen for the mRNA vaccine. Selection took account of the analysis of genetic divergence and the likelihood that a particular HA would be a threat to humans.
In mice and ferrets, vaccination elicited specific antibodies toward each of the 20 different HA targets in the vaccine. These findings highlight the flexibility of the mRNA vaccine platform without interference among the various mRNAs. Vaccination-protected mice and ferrets were exposed to both matched and mismatched viral strains.
Notably, lung infection of vaccinated ferrets with a matching virus strain was completely blocked, outperforming previous egg-based vaccines. Ferrets are more susceptible to human flu viruses than mice and exhibit a disease pathology and symptoms that are very similar to human infections.
According to Professor John Oxford, an eminent flu virologist, the new vaccine developed at the University of Pennsylvania represents a huge breakthrough. Conditional on the demonstration of its effectiveness in humans, a universal flu vaccine that could protect against all strains of the flu virus might be available in a few years.
Continued U.S. government grants are a prerequisite for Professor Scott Hensley, whose team at the Penn Institute of Immunology has the ambitious objective of giving people a baseline level of immune memory to diverse flu strains so that there will be far less disease and death when the next flu pandemic occurs.
But as with the mRNA COVID-19 vaccines, an infection may not be prevented by vaccination. The Penn team paper concludes optimistically that the overall approach will likely be useful for infectious diseases other than flu viruses.
The G7 Carbis Bay Health Declaration of July 12, 2021, pledged the G7 to work together to invest in innovation with the aim of making safe and effective vaccines, therapeutics, and diagnostics available within 100 days of the World Health Organization declaring a Public Health Emergency of International Concern.
The current production timeline of the standard egg-based flu vaccines is six months; too slow to stop the next flu pandemic virus within the targeted time frame of 100 days. By contrast, the universal mRNA vaccine could be scaled up quickly, albeit with the time required for regulatory approval.
This newly published innovation is an important step towards delivering on the G7 pledge. With the encouraging prospect of a universal flu vaccine in a few years, there is now a high chance that this will be available before the next flu pandemic emerges.
Find out more about RMS Infectious Diseases Model here.